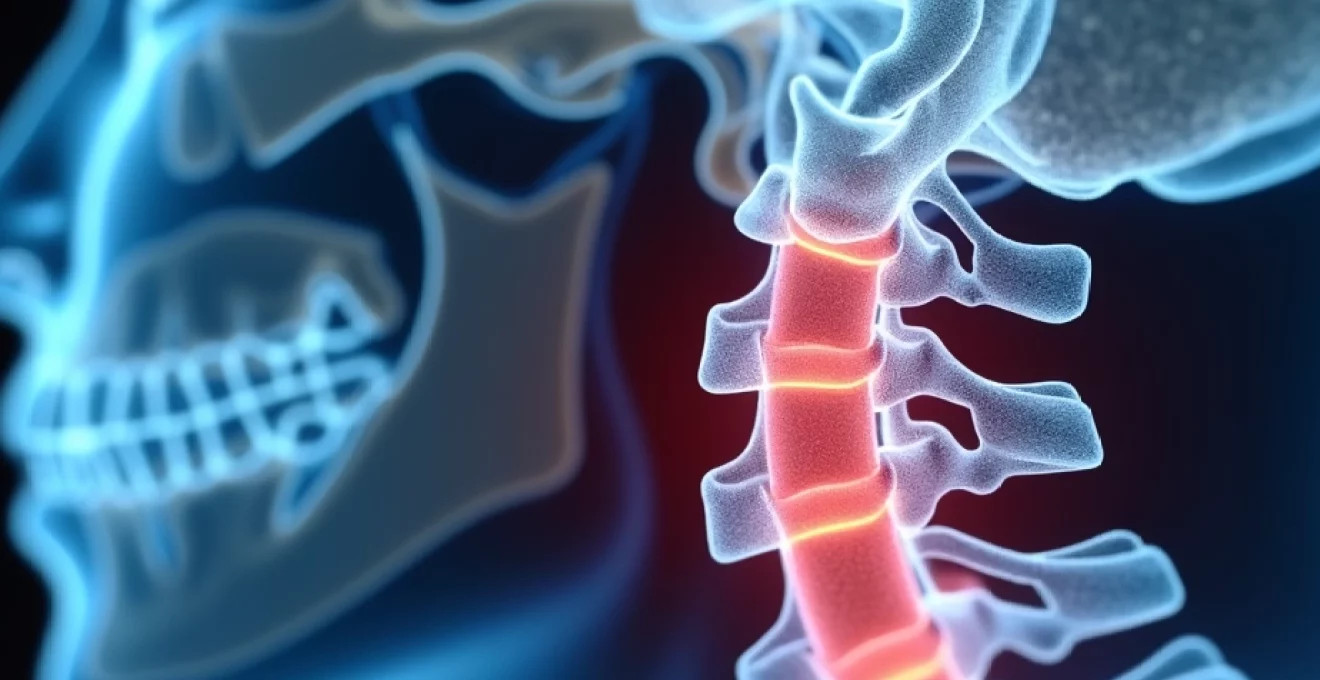
Cervical foraminal narrowing at the C6-C7 level represents one of the most clinically significant spinal conditions affecting the lower cervical spine. This degenerative process involves the gradual reduction of space within the neural foramina, the bony openings through which spinal nerve roots exit the spinal canal. The C6-C7 segment bears considerable mechanical stress due to its position at the junction between the mobile cervical spine and the more stable upper thoracic region, making it particularly susceptible to degenerative changes that can lead to neural compression and associated symptoms.
The prevalence of C6-C7 foraminal stenosis increases substantially with age, affecting approximately 40% of individuals over 60 years old and up to 75% of those aged 80 and older. However, the relationship between anatomical narrowing and clinical symptoms remains complex, with studies indicating that only 17.5% of patients with severe foraminal stenosis experience symptomatic radiculopathy. Understanding the intricate anatomy, pathophysiology, and treatment options for C6-C7 foraminal narrowing is essential for healthcare professionals managing patients with cervical spine disorders.
Anatomy of the C6-C7 cervical motion segment
Vertebral body structure and intervertebral disc characteristics
The C6-C7 motion segment exhibits unique anatomical characteristics that distinguish it from other cervical levels. The C6 vertebral body demonstrates a transitional morphology, featuring less prominent uncinate processes compared to upper cervical levels whilst maintaining substantial height and width dimensions. The C7 vertebra, often termed the vertebra prominens, serves as the anatomical landmark between the cervical and thoracic spine, characterised by its enlarged spinous process and rudimentary transverse processes that lack foramina transversaria.
The C6-C7 intervertebral disc represents the largest disc in the cervical spine, measuring approximately 5-6mm in height and exhibiting a more robust annular structure compared to upper cervical levels. This disc bears increased mechanical loading due to the weight of the head and the substantial range of motion at this level. The nucleus pulposus at C6-C7 maintains higher water content throughout early adulthood, but becomes increasingly susceptible to dehydration and degenerative changes with advancing age, contributing to disc height loss and subsequent foraminal narrowing.
Neural foramen dimensions and anatomical boundaries
The neural foramina at the C6-C7 level possess distinct anatomical boundaries that define the available space for neural structures. The superior boundary is formed by the pedicle of C6, whilst the inferior boundary consists of the pedicle of C7. The anterior border comprises the posterior aspect of the C6 vertebral body, the C6-C7 intervertebral disc, and the uncovertebral joint, also known as the joint of Luschka. The posterior boundary is established by the facet joint capsule and the ligamentum flavum.
Under normal physiological conditions, the C6-C7 neural foramen measures approximately 8-10mm in height and 4-5mm in width. These dimensions provide adequate space for the C7 nerve root, accompanying blood vessels, and surrounding connective tissue. However, degenerative changes affecting any of the surrounding structures can significantly compromise these dimensions, leading to neural compression. The funnel-shaped configuration of the foramen, with its narrowest point at the medial aspect, creates a natural zone of vulnerability where compression is most likely to occur.
Nerve root distribution and C7 radiculopathy patterns
The C7 nerve root, which exits through the C6-C7 neural foramen, follows a characteristic distribution pattern that enables clinicians to identify the source of compression based on symptom location. This nerve root provides motor innervation to the triceps muscle, wrist flexors, and finger extensors, whilst contributing to sensory innervation of the middle finger, index finger, and portions of the thumb. Understanding this anatomical distribution pattern proves crucial for accurate diagnosis and treatment planning.
When C7 radiculopathy develops secondary to foraminal narrowing, patients typically experience symptoms following specific dermatome and myotome patterns. The pain often radiates from the neck down through the posterior shoulder, posterior arm, and forearm, extending to the middle finger. Weakness may manifest as difficulty with triceps function, including reduced ability to perform push-up movements or lift objects away from the body. Sensory changes commonly affect the middle finger and adjacent digits, creating characteristic numbness and tingling patterns that correspond to the C7 dermatome distribution.
Facet joint configuration and uncovertebral joint mechanics
The C6-C7 facet joints demonstrate a transitional orientation between the more horizontal cervical facets and the steeper thoracic configuration. These joints, positioned at approximately 45 degrees to the transverse plane, provide stability whilst allowing for flexion, extension, lateral bending, and rotational movements. The joint surfaces are covered with hyaline cartilage and surrounded by a synovial joint capsule, which can undergo degenerative changes leading to capsular hypertrophy and osteophyte formation.
The uncovertebral joints at the C6-C7 level play a critical role in foraminal stenosis development. These structures, which are not true synovial joints but rather areas of contact between the uncinate processes of C7 and the lateral aspects of the C6 vertebral body, undergo progressive degenerative changes with age. Osteophyte formation at the uncovertebral joints represents a common cause of anterior foraminal encroachment, as these bony spurs can project directly into the neural foramen and compress the exiting nerve root.
Pathophysiology of C6-C7 foraminal stenosis
Degenerative disc disease and osteophyte formation
The pathophysiological cascade leading to C6-C7 foraminal stenosis typically begins with age-related degenerative disc disease. As the intervertebral disc loses proteoglycan content and water retention capacity, the disc height gradually decreases, leading to altered biomechanics throughout the motion segment. This height loss creates abnormal loading patterns on the facet joints and uncovertebral joints, accelerating degenerative changes in these structures.
Osteophyte formation represents the body’s attempt to stabilise the degenerative motion segment by increasing the surface area for load distribution. However, these bony projections frequently develop in locations that compromise neural foraminal space. Uncovertebral osteophytes commonly project posterolaterally into the anterior aspect of the neural foramen, whilst facet joint osteophytes may encroach from the posterior direction. The combination of disc height loss and osteophyte formation creates a pincer-like effect that progressively narrows the available space for neural structures.
Ligamentum flavum hypertrophy and capsular fibrosis
The ligamentum flavum, which forms the posterior boundary of the spinal canal and contributes to the posterior wall of the neural foramen, undergoes characteristic changes with advancing age and degenerative disease. This structure typically increases in thickness from an average of 2-3mm in younger individuals to 4-6mm or more in older adults with significant degenerative changes. The hypertrophy results from increased collagen deposition, elastin degradation, and fibroblast proliferation within the ligament structure.
Concurrent capsular fibrosis of the facet joints contributes to foraminal narrowing through thickening and contracture of the joint capsule. This process involves increased collagen production, inflammatory cell infiltration, and gradual replacement of normal capsular tissue with dense fibrous tissue. The combination of ligamentum flavum hypertrophy and capsular fibrosis creates significant posterior compression of neural structures, particularly when combined with anterior osteophyte formation and disc bulging.
Facet joint arthropathy and synovial cyst development
Progressive facet joint arthropathy at the C6-C7 level follows a predictable pattern of cartilage degradation, subchondral bone changes, and osteophyte formation. The process begins with mechanical wear of the articular cartilage due to abnormal loading patterns resulting from disc degeneration. As cartilage thickness decreases, the underlying subchondral bone experiences increased stress, leading to sclerosis, cyst formation, and eventual osteophyte development along the joint margins.
Synovial cyst formation, whilst less common in the cervical spine compared to the lumbar region, can contribute to foraminal stenosis when present. These cysts develop as a result of synovial fluid herniation through defects in the joint capsule, creating space-occupying lesions that can compress neural structures. The cysts may undergo inflammatory changes, further increasing their volume and potential for neural compression. Additionally, the presence of synovial cysts often indicates advanced facet joint degeneration and may be associated with segmental instability.
Inflammatory cascade and neural compression mechanisms
The development of neural symptoms in C6-C7 foraminal stenosis involves complex interactions between mechanical compression and inflammatory processes. Mechanical compression of the nerve root triggers a cascade of inflammatory mediators, including tumour necrosis factor-alpha, interleukin-1, and prostaglandin E2. These substances increase vascular permeability, promote oedema formation, and sensitise nociceptors, contributing to pain generation and maintenance.
The confined space within the neural foramen creates a compartment syndrome-like environment where even small increases in tissue volume can result in significant pressure elevations. This increased pressure compromises neural blood flow, leading to ischaemia and further inflammatory mediator release. The resulting cycle of inflammation, oedema, and ischaemia can perpetuate symptoms even when the initial mechanical compression is relatively mild, explaining why some patients experience severe symptoms with minimal anatomical narrowing visible on imaging studies.
Clinical manifestations and C7 radicular symptoms
The clinical presentation of C6-C7 foraminal stenosis demonstrates considerable variability, ranging from asymptomatic anatomical narrowing to severe, debilitating radicular pain and neurological dysfunction. The hallmark symptom involves neck pain that radiates into the shoulder, arm, and hand following the C7 dermatome distribution. This pain typically exhibits a sharp, burning, or electric-like quality and may be accompanied by deep, aching sensations in the affected muscles.
Patients frequently report symptom exacerbation with specific neck movements, particularly extension and ipsilateral lateral bending, which further narrow the neural foramen and increase nerve root compression. The Spurling test, involving neck extension with lateral bending and axial compression towards the affected side, often reproduces symptoms and serves as a valuable diagnostic manoeuvre. Conversely, neck flexion and contralateral lateral bending may provide symptom relief by opening the neural foramen and reducing compression.
Motor symptoms associated with C7 radiculopathy include weakness of the triceps muscle, resulting in difficulty with pushing movements and overhead activities. Patients may notice reduced grip strength, particularly affecting finger extension and wrist flexion. Fine motor control can be impaired, leading to difficulty with tasks requiring precise hand coordination, such as writing or manipulating small objects. Muscle atrophy may become apparent in chronic cases, particularly affecting the triceps and forearm muscles innervated by the C7 nerve root.
Sensory manifestations typically involve numbness, tingling, and altered sensation affecting the middle finger, index finger, and portions of the thumb. These symptoms may follow a glove-like distribution and can vary in intensity throughout the day. Some patients experience hyperaesthesia or allodynia, where normally non-painful stimuli become uncomfortable or painful. Sleep disturbances are common due to positional factors that affect foraminal dimensions during recumbent positioning.
The relationship between imaging findings and clinical symptoms in cervical foraminal stenosis remains complex, with significant anatomical narrowing sometimes occurring in the absence of symptoms, whilst severe radicular pain may develop with relatively mild imaging changes.
Advanced imaging protocols for foraminal assessment
MRI sequences and T2-Weighted sagittal imaging analysis
Magnetic resonance imaging serves as the gold standard for evaluating C6-C7 foraminal stenosis, providing detailed visualisation of both bony and soft tissue structures surrounding the neural foramen. T2-weighted sagittal sequences offer optimal visualisation of the neural foramina by demonstrating the bright signal intensity of cerebrospinal fluid within the foraminal space. Normal foramina appear as bright, teardrop-shaped openings, whilst stenotic foramina demonstrate reduced signal intensity due to encroachment by degenerative tissues.
The sagittal T2-weighted sequences enable assessment of foraminal height, which typically decreases in the presence of disc space narrowing and osteophyte formation. Grading systems utilise the relationship between foraminal cerebrospinal fluid signal and nerve root visibility to classify stenosis severity. Grade 0 indicates normal foraminal dimensions with abundant cerebrospinal fluid signal, Grade 1 shows mild narrowing with reduced but present cerebrospinal fluid signal, Grade 2 demonstrates moderate narrowing with minimal cerebrospinal fluid signal, and Grade 3 represents severe stenosis with complete obliteration of cerebrospinal fluid signal and nerve root compression.
Axial T2-weighted sequences provide complementary information regarding the anteroposterior dimensions of the neural foramen and help identify specific structures causing compression. These images excel at demonstrating uncovertebral osteophytes, facet joint hypertrophy, and ligamentum flavum thickening. The combination of sagittal and axial imaging enables comprehensive three-dimensional assessment of foraminal anatomy and pathology, facilitating accurate diagnosis and treatment planning.
CT myelography indications and contrast enhancement patterns
Computed tomography myelography represents an alternative imaging modality for patients who cannot undergo MRI due to contraindications such as pacemakers, cochlear implants, or severe claustrophobia. This technique involves intrathecal injection of iodinated contrast material followed by CT imaging, providing excellent visualisation of neural structures and their relationship to surrounding bony anatomy. The contrast material outlines the nerve root sleeves, enabling detection of compression or displacement that may not be apparent on conventional CT imaging.
The enhanced contrast resolution of CT myelography proves particularly valuable for distinguishing between disc herniation and osteophyte formation as causes of foraminal stenosis. Bony osteophytes appear as discrete, hard-edged densities that indent or displace the contrast-filled nerve root sleeve, whilst disc material typically demonstrates softer, more irregular margins. The technique also provides superior visualisation of calcified structures, including calcified disc herniations and ossified ligaments that may contribute to neural compression.
Post-myelography CT imaging can reveal dynamic changes in foraminal dimensions with positional variations. Extension and flexion positioning during image acquisition may demonstrate positional narrowing or widening of the neural foramen, providing valuable information regarding the functional significance of anatomical changes. This dynamic assessment capability makes CT myelography particularly useful for evaluating patients with positional symptoms or when surgical intervention is being considered.
Oblique radiographic views and foraminal grading systems
Oblique radiographic views, obtained at 45-degree angles to the sagittal plane, provide direct visualisation of the neural foramina and remain valuable screening tools for foraminal stenosis assessment. These views demonstrate the bony boundaries of the neural foramen, including the pedicles, vertebral bodies, and facet joints. Normal foramina appear as well-defined, oval-shaped openings, whilst stenotic foramina show irregular narrowing, sclerosis, or complete obliteration of the foraminal space.
Several grading systems have been developed to standardise the assessment of foraminal stenosis severity on oblique radiographs. The most commonly used system classifies stenosis into four grades based on the degree of foraminal narrowing: Grade 0 indicates normal foraminal dimensions, Grade 1 shows mild narrowing with preservation of foraminal shape, Grade 2 demonstrates moderate narrowing with altered foraminal contour, and Grade 3 represents severe stenosis with marked deformity or near-complete obliteration of the foraminal space.
Whilst oblique radiographs provide limited soft tissue detail compared to MRI, they offer several advantages including widespread availability, lower cost, and ability to assess multiple levels simultaneously. These views prove particularly useful for monitoring progression of degenerative changes over time and for evaluating bony fusion following surgical intervention. The combination of oblique radiographs with advanced imaging modalities provides comprehensive assessment of foraminal pathology.
Conservative treatment modalities and interventional techniques
Selective nerve root blocks and epidural steroid injections
Selective nerve root blocks represent a cornerstone of interventional pain management for C6-C7 foraminal stenosis, offering both diagnostic and therapeutic benefits. These procedures involve the precise injection of local anaesthetic and corticosteroid medications directly around the affected C7 nerve root under fluoroscopic or CT guidance. The diagnostic component helps confirm the specific nerve root as the pain generator, whilst the therapeutic aspect provides anti-inflammatory effects that can reduce neural oedema and improve symptoms for weeks to months.
The technique requires meticulous attention to anatomical landmarks and needle positioning to ensure accurate medication delivery whilst avoiding vascular structures and the spinal cord. A posterior approach is typically employed, with the needle directed through the posterior aspect of the neural foramen to reach the nerve root sleeve. Pre-procedural contrast injection confirms proper needle positioning and helps identify vascular uptake, which must be avoided to prevent complications such as spinal cord infarction or seizures.
Cervical epidural steroid injections offer an alternative approach for patients with multilevel pathology or when selective nerve root blocks are technically challenging. These procedures involve injection of corticosteroids into the epidural space, allowing medication to bathe multiple nerve roots simultaneously. The interlaminar approach at C6-C7 or C7-T1 levels provides access to the epidural space whilst minimising risk to neural structures. Studies demonstrate success rates of 60-80% for symptom improvement lasting 3-6 months when appropriate patient selection criteria are employed.
Cervical traction protocols and manual therapy approaches
Cervical traction represents a time-tested conservative treatment modality that aims to increase foraminal dimensions through distraction of the vertebral segments. The mechanism involves gentle longitudinal force application that separates the vertebrae, theoretically increasing neural foraminal space and reducing nerve root compression. Modern traction protocols utilise intermittent traction with forces ranging from 10-15 pounds initially, progressing to 20-25 pounds based on patient tolerance and symptom response.
Manual cervical traction performed by skilled physiotherapists allows for real-time assessment of patient response and immediate adjustment of force magnitude and direction. The therapist can apply specific vectored forces to target the affected motion segment whilst monitoring for symptom reproduction or relief. This personalised approach enables optimisation of treatment parameters and helps identify patients most likely to benefit from home traction units or other mechanical traction devices.
Manual therapy techniques encompass a broader range of interventions including mobilisation, manipulation, and soft tissue techniques. Cervical mobilisation involves passive movement of the cervical spine through physiological and accessory ranges of motion, aiming to restore normal joint mechanics and reduce muscle guarding. Grade III and IV mobilisations applied to the C6-C7 segment can help improve segmental mobility and may reduce symptoms through neurophysiological pain modulation mechanisms. Soft tissue techniques targeting the suboccipital muscles, upper trapezius, and levator scapulae help address secondary muscle dysfunction that often accompanies cervical radiculopathy.
Neuropathic pain management and gabapentinoid therapy
The neuropathic component of C6-C7 radicular pain requires specific pharmacological approaches that target altered neural processing mechanisms. Gabapentinoids, including gabapentin and pregabalin, represent first-line treatments for neuropathic pain associated with cervical foraminal stenosis. These medications work by binding to voltage-gated calcium channels in the central nervous system, reducing excitatory neurotransmitter release and dampening abnormal neural transmission that characterises neuropathic pain states.
Gabapentin dosing typically begins at 300mg daily, with gradual titration to therapeutic levels of 1800-3600mg daily divided into three doses. The slow titration schedule helps minimise common side effects including dizziness, sedation, and peripheral oedema. Pregabalin offers advantages of twice-daily dosing and potentially faster onset of action, with typical dosing ranging from 150-600mg daily. Both medications demonstrate similar efficacy profiles, with 30-50% of patients experiencing clinically meaningful pain reduction when used as part of multimodal treatment approaches.
Tricyclic antidepressants, particularly amitriptyline and nortriptyline, provide alternative options for neuropathic pain management when gabapentinoids are contraindicated or ineffective. These medications work through multiple mechanisms including sodium channel blockade, norepinephrine reuptake inhibition, and NMDA receptor antagonism. Starting doses of 10-25mg at bedtime help minimise anticholinergic side effects whilst providing analgesic benefits. The sedating properties of tricyclic antidepressants can be advantageous for patients experiencing sleep disturbances related to their radicular symptoms.
Surgical interventions for refractory C6-C7 foraminal stenosis
Anterior cervical discectomy and fusion (ACDF) techniques
Anterior cervical discectomy and fusion represents the most commonly performed surgical procedure for C6-C7 foraminal stenosis when conservative treatments fail to provide adequate symptom relief. This approach offers excellent visualisation of the neural foramen from an anterior perspective, allowing comprehensive decompression of the nerve root whilst maintaining cervical lordosis and stability. The procedure involves complete removal of the intervertebral disc, followed by decompression of osteophytes and other compressive structures, then placement of an interbody graft or cage to restore disc height and promote fusion.
The surgical technique begins with a standard anterior cervical approach, typically through a transverse incision at the appropriate level. Careful dissection through fascial planes provides access to the anterior spine whilst preserving vital structures including the recurrent laryngeal nerve and oesophagus. Complete discectomy enables direct visualisation of the posterior longitudinal ligament and identification of compressive pathology. Removal of uncovertebral osteophytes requires particular attention to avoid injury to the vertebral artery, which courses in close proximity to these structures.
Modern ACDF techniques emphasise restoration of normal cervical alignment and maintenance of appropriate disc height to optimise foraminal dimensions. Structural allografts, autografts, or titanium cages filled with bone graft material provide immediate mechanical support whilst promoting long-term fusion. Anterior cervical plating enhances fusion rates and provides immediate stability, allowing for earlier mobilisation and potentially improved outcomes. Studies demonstrate fusion rates exceeding 95% with modern techniques, with excellent or good clinical outcomes reported in 85-90% of patients with isolated C6-C7 foraminal stenosis.
Posterior cervical foraminotomy and laminoplasty procedures
Posterior cervical foraminotomy offers a motion-preserving alternative to anterior fusion procedures, particularly suitable for patients with isolated foraminal stenosis without significant anterior pathology. This technique involves removal of the medial portion of the facet joint and lamina to enlarge the neural foramen whilst preserving the intervertebral disc and motion segment function. The approach proves especially beneficial for younger patients who wish to maintain cervical mobility and avoid the potential complications associated with fusion procedures.
The surgical procedure utilises a posterior midline approach with muscle-splitting techniques to minimise soft tissue trauma. Identification of the appropriate level requires careful attention to anatomical landmarks, often aided by intraoperative imaging confirmation. The key steps involve removal of the inferior aspect of the C6 lamina and the superior portion of the C7 lamina, followed by medial facetectomy to expose and decompress the C7 nerve root. Preservation of greater than 50% of the facet joint maintains segmental stability whilst providing adequate decompression.
Minimally invasive posterior foraminotomy techniques utilise tubular retractor systems and endoscopic visualisation to achieve neural decompression through smaller incisions. These approaches reduce muscle trauma, decrease postoperative pain, and facilitate faster recovery compared to traditional open procedures. However, the learning curve for minimally invasive techniques is substantial, and patient selection criteria must be carefully applied to ensure optimal outcomes. Long-term studies demonstrate symptom relief in 80-85% of patients whilst preserving motion at the treated level.
Cervical laminoplasty represents another motion-preserving option for patients with multilevel stenosis or concurrent central canal narrowing. This technique involves creating a hinged opening of the posterior arch to enlarge the spinal canal whilst maintaining posterior column integrity. Open-door laminoplasty creates a unilateral hinge with contralateral opening, whilst double-door techniques create bilateral openings. Although primarily designed for central stenosis, laminoplasty can provide indirect foraminal decompression through restoration of cervical lordosis and reduction of ligamentum flavum buckling.
Artificial disc replacement and motion preservation strategies
Cervical artificial disc replacement has emerged as an innovative motion-preserving alternative to fusion for carefully selected patients with C6-C7 foraminal stenosis. This technology aims to maintain normal segmental motion whilst providing neural decompression, potentially reducing the risk of adjacent segment disease that can occur following fusion procedures. The ideal candidate demonstrates single-level pathology with preserved facet joints, adequate bone quality, and absence of significant cervical instability or deformity.
The surgical technique mirrors that of ACDF through the initial approach and discectomy phases, but diverges with the placement of a motion-preserving prosthetic device rather than a fusion construct. Precise sizing and positioning of the artificial disc prove critical for optimal biomechanical function and longevity. The prosthetic devices typically consist of metal endplates with polyethylene or metal-on-metal bearing surfaces designed to replicate normal cervical motion patterns. Maintenance of appropriate cervical lordosis and disc height restoration remain paramount to achieving successful foraminal decompression.
Clinical studies comparing cervical disc replacement to ACDF demonstrate similar rates of neural decompression and symptom relief, with potential advantages in preservation of cervical motion and reduced adjacent segment degeneration. However, long-term data regarding implant durability and revision rates continue to evolve. Contraindications include significant facet joint arthropathy, osteoporosis, active infection, and cervical instability. The learning curve for artificial disc implantation requires specialised training and experience to achieve optimal outcomes whilst minimising complications such as implant migration or subsidence.
Hybrid constructs combining fusion and artificial disc replacement at adjacent levels represent an emerging strategy for multilevel cervical pathology. This approach allows for customised treatment of each motion segment based on specific pathological characteristics whilst maintaining some degree of cervical mobility. The C6-C7 level frequently serves as a fusion level in hybrid constructs due to its transitional anatomy and higher loads compared to upper cervical segments. Long-term studies will be necessary to determine the optimal application of these motion preservation strategies in the management of cervical foraminal stenosis.